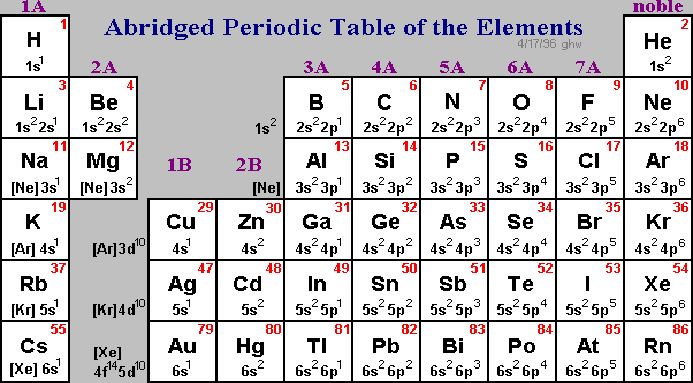
Determining Electron Configuration
One of the skills you will need to learn to succeed in freshman chemistry is being able to determine the electron configuration of an atom. An electron configuration is basically an account of how many electrons there are, and in what orbitals they reside under "normal" conditions. For example, the element hydrogen (H) has one electron. We know this because its atomic number is one (1), and the atomic number tells you the number of electrons. Where does this electron go? The one electron of hydrogen goes into the lowest energy state it possibly can, which means it will start at "level" one and goes into "s" orbitals first. We say that hydrogen has a "[1s1]" electron configuration. Looking at the next element on the Periodic Table --helium, or He -- we see it has an atomic number of two, so two electrons. Since " s" orbitals can hold up to two electrons, helium has an electron configuration of "[1s2]".What about larger atoms? Let's look at carbon, with an atomic number of 6. Where do its 6 electrons go?
- First two: 1s2
- Next two: 2s2
- Last two: 2p2
We can therefore say that carbon has the electron configuration of "[1s22s22p2]".
The table below shows the subshells, the number of orbitals, and the maximum number of electrons allowed:
| Subshell | Number of Orbitals | Maximum Number of Electrons |
|---|---|---|
| s | 1 | 2 |
| p | 3 | 6 |
| d | 5 | 10 |
| f | 7 | 14 |
Sometimes you will see the notation: "[Ne]3s23p6", which means to include everything that is in neon (Ne, 10) plus the stuff in the "3"-level orbitals.

No comments:
Post a Comment