Introduction
Massive atomic nuclei are born naked, but their net positive charge, Z, quickly attracts Z comparatively mass-less electrons to produce neutral atoms.
- The nucleus of a neon atom – atomic number 10 and so the Ne10+ ion – attracts 10 negatively charged electrons to give an atom of neon.
The negatively charged electrons do not nuclear react with the positively charged protons of the nucleus, as they do inside neutron stars, instead they associate as three dimensional resonant standing waves.
The electrons move about the point positive charge
in a beautiful and subtle quantum mechanical dance
- The modes of resonance for single electron systems such as the hydrogen atom are described by the Schrödinger wave equation.
- Schrödinger knew of de Broglie's proposal that a moving particle has wavelength, l, proportional to Plank's constant, h, and momentum p so that l = h/p, a property we now known as wave-particle duality.
- Schrödinger constructed a differential equation for a wavelike electron resonating in three dimensions about a point positive charge (Wikipedia):
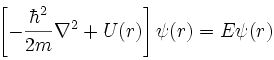
- Solutions to the Schrödinger wave equation correspond to modes of electron resonance and are formally called wavefunctions.
- Wavefunctions assume discrete, or quantised, energies and have energies which correspond to the spectral lines of one electron atoms and ions of the type: H•, He+, Li2+, Be3+, etc.
- Although not exactly the same, chemists tend to call wavefunctions "orbitals".
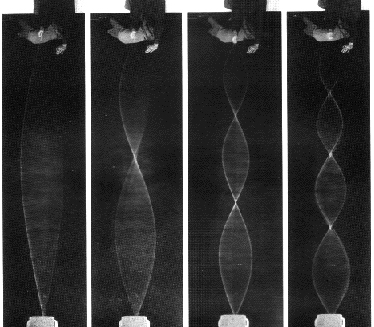
1-Dimensional resonant standing waves, the vibrating string:
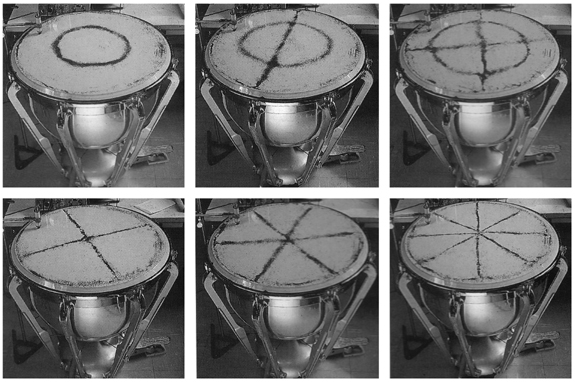
2-Dimensional resonant standing wave, a vibrating drum skin (see QuickTime movies here andhere):
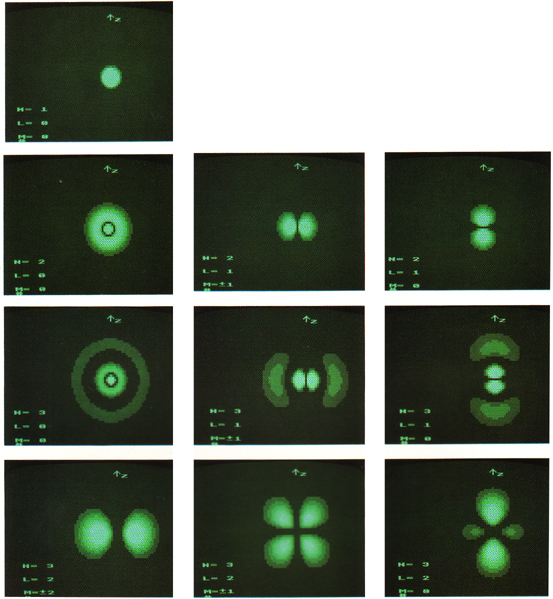
3-Dimensional resonant standing waves, atom wavefunctions from the Schrödinger wave equation:

No comments:
Post a Comment