Quantum Numbers to Orbitals
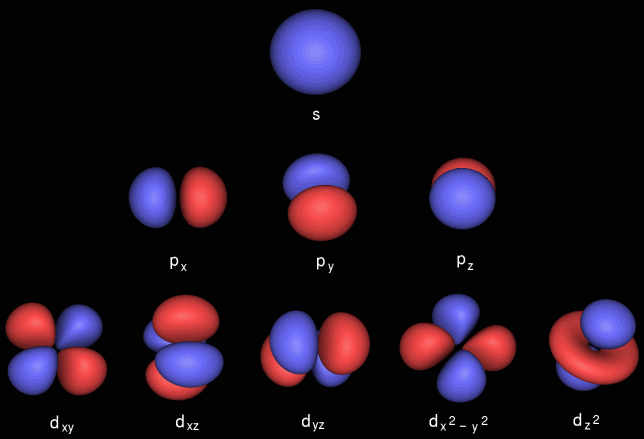
Chemists recognise s, p, d and f-orbitals. The topologies of these orbitals: the shape, phase & electron occupancy are described by four quantum numbers:
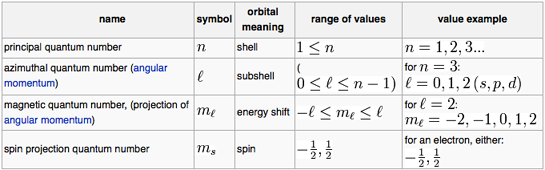
n | The principal quantum number |
l | The subsidiary or azimuthal or angular momentum or orbital shape quantum number |
ml | The magnetic quantum number |
ms | The electron spin quantum number |
From Wikipedia:
These quantum numbers conspire to give spherical s-orbitals, dumbbell shaped p-orbitals that come in sets of three, double dumbbell d-orbitals that come in sets of five, etc.:
Electrons enter and fill orbitals according to four rules:
Pauli Exclusion Principal | Orbitals can contain a maximum of two electrons which must be of opposite spin. |
Aufbau or Build-up Principle | Electrons enter and fill lower energy orbitals before higher energy orbitals. |
Hund's Rule | When there there are degenerate (equal energy) orbitals available, electrons will enter the orbitals one-at-a-time to maximise degeneracy, and only when all the orbitals are half filled will pairing-up occur. This is the rule of maximum multiplicity. |
Madelung's Rule | Orbitals fill with electrons as n + l, where n is the principal quantum number and l is the subsidiary quantum number. This rule 'explains' why the 4s orbital has a lower energy than the 3d orbital, and it gives the periodic table its characteristic appearance. |
Certain 'magic' numbers of electrons of electrons exhibit energetic stability: 2, 10, 18, 36, 54, 86 and,one assumes, 118, are associated with the Group 18 inert or noble gases: He, Ne, Ar, Kr, Xe, Rn & Uuo.
- The 'magic' numbers inevitably arise from the underlying quantum mechanics, but as Richard Feynman told us (here): "I think I can safely say that nobody understands quantum mechanics."
We can predict quantum mechanical patterns, but we don't know why we can predict the patterns. We do not understand QM in terms of a deeper theory.
Now available on the web are Richard Feynman's "Messenger Lectures" where he looks at the nature of physical theory and its relationship with mathematics. Highly recommended!
Quantum Patterns
The pattern of orbital structure is very rich and can be mapped onto the two dimensions of paper in many different ways.
Some mappings emphasize how the orbitals are ordered and filled with electrons, others stress how the chemical elements and their orbitals are ordered with respect to atomic number Z. Each tells us something different about atomic orbital structure and/or elemental periodicity.
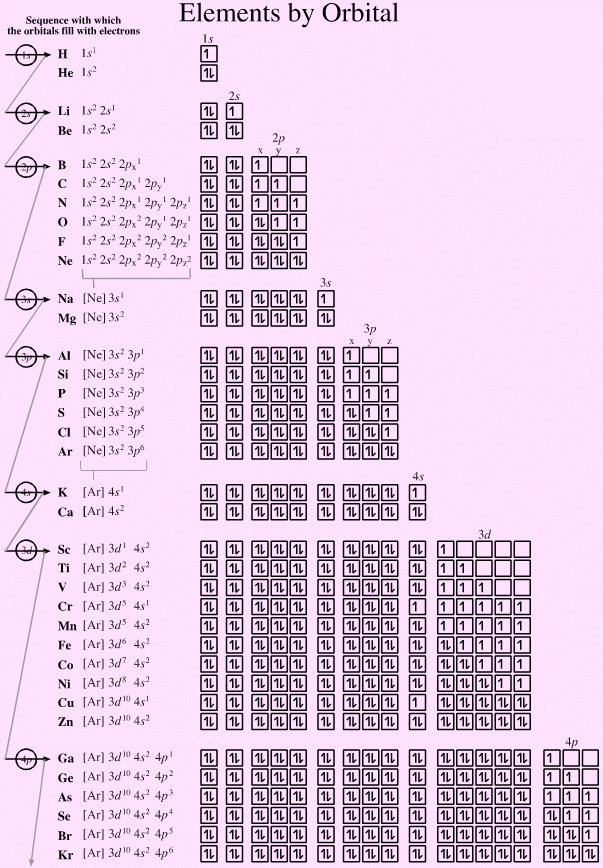
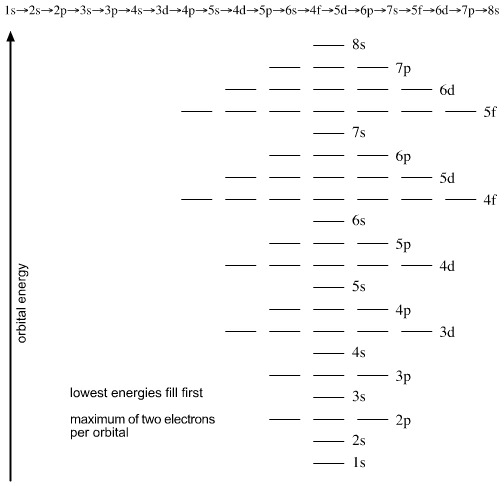
Orbital Filling
The sequence of orbital filling is, from the bottom of this diagram, upwards:
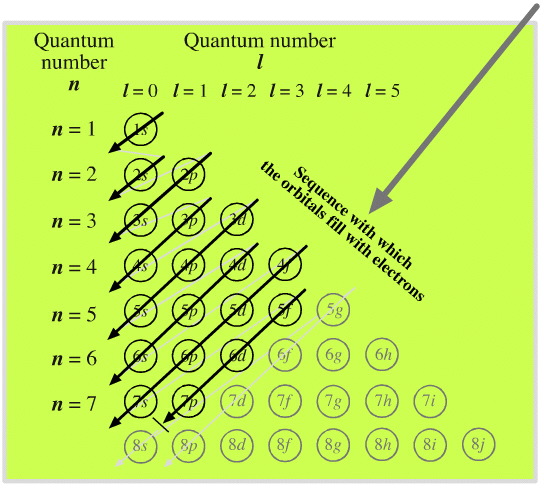
Electron Shells
As electrons are added, the quantum numbers build up the orbitals. Read this diagram, from the top downwards:
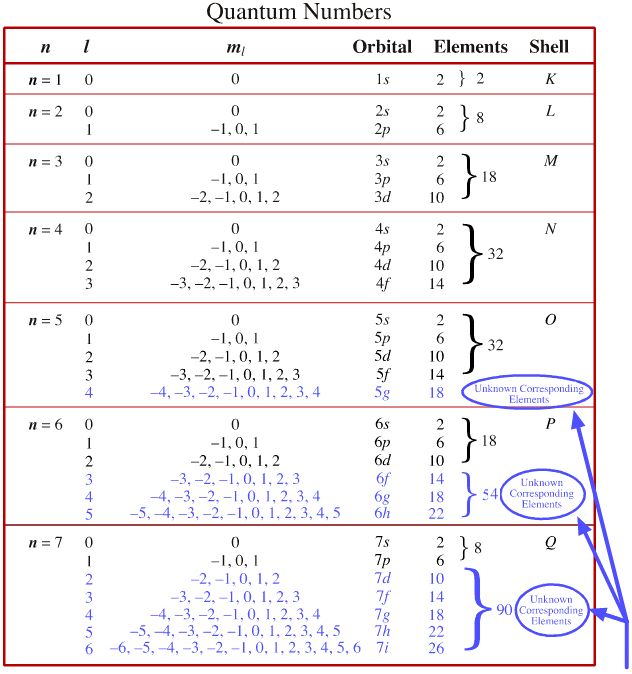
Madelung's Rule says the orbitals fill in the order n + l (lowest first). This gives the sequence:
- (n = 1) + (l = 0) = 1 1s
- (n = 2) + (l = 0) = 2 2s
- (n = 2) + (l = 1) = 3 2p
- (n = 3) + (l = 0) = 3 3s
- (n = 3) + (l = 1) = 4 3p
- (n = 4) + (l = 0) = 4 4s
- (n = 3) + (l = 1) = 4 3d
- (n = 4) + (l = 1) = 5 4p
- (n = 5) + (l = 0) = 5 5s
- and so on...
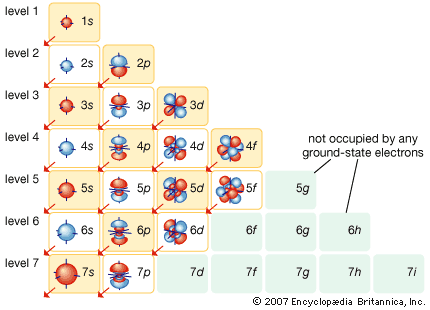
A nice electron shell representation from the Encyclopedia Britannica:
Elements by Orbital, And Some Subtleties...
The electronic structure can be illustrated adding electrons to boxes (to represent orbitals). This representation shows the Pauli exclusion principle, the aufbau principle and Hund's rule in action.
There are some subtle effects with the d block elements chromium, Cr, and copper, Cu. Hund's rule of maximum multiplicity lowers the energy of the 3d orbital below that of the the 4s orbital, due to the stabilisation achieved with a complete and spherically symmetric set of five 3d orbitals containing five or ten electrons. Thus,
- Chromium has the formulation: [Ar] 3d5 4s1 and not: [Ar] 3d4 4s2
- Copper has the formulation: [Ar] 3d10 4s1 and not: [Ar] 3d9 4s2

No comments:
Post a Comment